Water Soluble Fertilizer Calculator
The water soluble fertilizer calculator (also a fertilizer ppm calculator or a hydroponic nutrient formula calculator) generates a recipe for a water-soluble fertilizer solution.
Learning how to mix fertilizer solutions is one of the essential fertilizer calculations for greenhouse crops and indoor plant factories. Use our calculator to save time and money, as well as growing great plants!
For calculating how much dry fertilizer you need to apply to grass or crops, check out our fertilizer calculator.
Why mix your own fertilizer solution?
Whether you are a greenhouse grower or a hobbyist, there are numerous benefits to be had from learning how to make fertilizer solutions, stock solutions, or hydroponic nutrients. By using the fertilizer mixing calculator, you can:
-
Save money. Mixing nutrient solution is often less expensive than pre-made nutrient solutions.
-
Transport and store fertilizer in larger quantities. Dry fertilizers take up less space than liquid fertilizers, weigh less, and can be stored safely for extended periods of time before being made into a liquid.
-
Gain complete control over nutrient composition. By mixing a custom solution, you gain control over every macro- and micronutrient your plant will get.
-
Ensure consistent, healthy plants. By correctly calculating your nutrient formula, you will help ensure a consistent growing environment for plants, leading to better results.
-
Be satisfied! By learning to mix fertilizer solution, you will become a more knowledgeable horticulturist and know exactly what you are feeding your plants.
Water soluble fertilizer — a guide to the label
There are two important components of a water-soluble fertilizer's label that you need to be aware of:
- The N-P-K numbers; and
- The "guaranteed analysis".
The N-P-K numbers are the three large numbers you'll often see on the front of the packaging, such as 20-8-20. These numbers represent the three primary nutrients (nitrogen, phosphorus, and potassium) as a percent of the fertilizer's weight. The nutrients are present as nitrogen (N), phosphate (P2O5), and potash (K2O). In the example 20-8-20 fertilizer, the label tells you that:
- 20% of the bag's weight is composed of nitrogen;
- 8% of the weight is composed of phosphate; and
- 20% of the weight is composed of potash.
The remainder of the weight is comprised of other elements or filler.
Next, look for a small list, often on the back of the bag, titled guaranteed analysis. It will look something like this:
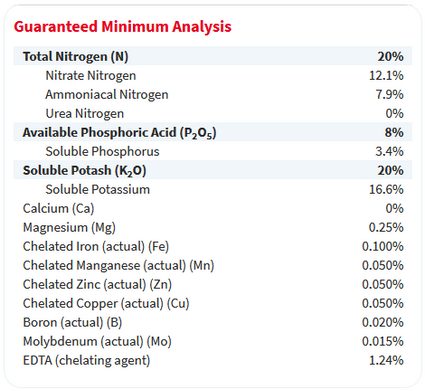
The guaranteed analysis label also describes the percentage of the fertilizer's weight that comprises each nutrient. Notice that the percentages for nitrogen, phosphate (here, it's called phosphoric acid), and potash match the 20-8-20 label on the front.
However, keep in mind that the weights of the elements phosphorus (P) and potassium (K) are less than the weights of the molecules phosphate and potash, which include the weight of oxygen. This distinction becomes important when you need to calculate a fertilizer recipe with a specific concentration of P or K.
How to use the water soluble fertilizer calculator
Making hydroponic nutrients becomes much easier with the water-soluble fertilizer calculator! All you need to do is follow these instructions:
-
Enter the N-P-K numbers or the elemental percentages found under "guaranteed analysis" on the fertilizer's label.
-
Select the desired element for which you have a target concentration (in ppm or mmol per liter) in mind.
-
Enter the desired concentration, in either weight concentration (such as ppm) or molar concentration (such as mM or mmol/L).
-
Adjust the final volume of diluted solution to the size of your mixing container.
-
Record how much dry fertilizer is required to achieve your target concentration.
-
See the table below the calculator to find out how much of each element your recipe will add.
Calculating a nutrient solution recipe to achieve 200 ppm nitrogen
What if you want to find out the hydroponic nutrients by hand? Let's say you have a 100-L water tank and want to calculate how much fertilizer to add to achieve 200 ppm nitrogen (a common recommendation for many greenhouse crops). For this example, we'll be using a soluble fertilizer with 20-8-20 on the label. From the label, we know that our fertilizer is comprised of 20% nitrogen, 8% phosphate, and 20% potash by weight. Follow these steps to find the nutrient solution recipe:
1. Rewrite ppm as mg/L
You should know that ppm is the same as mg/L (mass of solute per volume of water). So, rewrite the units of the desired concentration of nitrogen:
nitrogen concentration (mg/L) = 200 ppm = 200 mg/L nitrogen
2. Determine final fertilizer concentration
Now we know how many mg/L of nitrogen we should add to water. To find how many mg/L of fertilizer to add, divide the nitrogen concentration by the percent weight of N. The formula is:
fertilizer concentration (mg/L) = nitrogen concentration (mg/L) / percent weight
Percent weight is read from the label and can be written as a decimal (20% = 0.2) or as 20 g N / 100 g fertilizer. Inserting our values, we get:
fertilizer concentration (mg/L) = 200 mg/L N / 0.2 = 1000 mg/L = 1 g/L
3. Calculate the weight of fertilizer to be added
You can now use the fertilizer concentration of 1 g/L as a mixing ratio for any volume of water using the formula:
fertilizer (g) = fertilizer concentration (g/L) × water volume (L)
Since we are using a 100-L water tank, the calculation is as follows:
fertilizer (g) = 1 g/L of fertilizer × 100 L = 100 g
Therefore, we should add 100 g fertilizer to 100 L of water to achieve 200 ppm N.
4. Bonus step: Calculate concentrations of other elements
Since the fertilizer concentration is 1 g/L, you can use the equation in Step 2 to calculate the resulting ppm of P2O5 and K2O using the percent weights of P2O5 and K2O. The rearranged equations are:
P2O5 concentration = fertilizer concentration × percent weight of P2O5
and
K2O concentration = fertilizer concentration × percent weight of K2O
and so on for any other fertilizer components. However, you must still convert the compounds P2O5 and K2O to elemental concentrations of P and K. To do this, you will need to use the percent weight of P in P2O5 and the percent weight of K in K2O. The conversions are as follows:
weight of P = weight of P2O5 / 2.29133
and
weight of K = weight of K2O / 1.20460
If you're keen to practice, you can look up the atomic weights of each element to calculate this conversion yourself using the standard atomic weights of P, K, and O (retrieved from the 2021 Standard Atomic Weights page on the ):
Element | Atomic weight (g/mol) |
|---|---|
Nitrogen (N) | 14.007 |
Phosphorus (P) | 30.973761998 |
Potassium (K) | 39.0983 |
Oxygen (O) | 15.999 |
For example, you can find the weight conversion from K to K2O by dividing the atomic weight of all potassium atoms (2 in this case) by the total weight of K2O (2 potassium atoms + 1 oxygen atom). Alternatively, you can use our percent composition calculator.
A simple method to estimate the volume of your container
Before you can calculate fertilizer weight, you need to know the volume of your mixing container. If your mixing tank is very close to being a perfect cylinder or simple shape, you can estimate it reasonably well using our tank volume calculator. However, inaccuracies could arise if your tank has rounded edges or if you don't correctly account for wall thickness.
Another method for irregularly-shaped containers is to use a smaller vessel of a known volume, such as a bucket or a 1-gallon jug, to fill up your mixing tank. Periodically make gradation marks on the wall of the container or on a stick to indicate the volume added. For example, if you fill your water tank with a 1-gallon jug, you might make a mark every 5 gallons.
If you have multiple mixing containers, it is best to measure them all using the same bucket or jug so that if there is any inaccuracy, they will still be consistent with each other.
What water source should you use?
If your growing setup recirculates irrigation water, you must be cautious of the salt levels of your water source, as salinity (sodium chloride or NaCl) can build over time and harm plant growth.
Rainwater is a good option for hydroponics as you won't have to worry about the initial salt concentration, and it can be collected from greenhouse roofs.
Deionized water also provides a high level of control and consistency. However, you may have to add more minerals or micronutrients that would otherwise be present in tap water.
In other horticultural scenarios, tap water or groundwater may be suitable and convenient, depending on the local water quality. The benefit of municipal water is that it may already contain calcium and other nutrients, meaning that you wouldn't need to add these when mixing fertilizer solutions. However, be aware that water quality can change from season to season. In some regions, the water may contain too much salt for irrigation water to be recirculated.
If you are mixing stock solutions, tap water or groundwater would likely be a poor choice because the calcium in the water may precipitate with sulfates and phosphates at high concentrations.
If you are interested in using tap water or groundwater, it is essential to know your water's composition. Here are a few tips:
-
Get a water analysis report if it's available. Check with your city's water department; many cities keep records of the mineral content of public water. Alternatively, you can send a water sample to a lab for analysis.
-
Regularly measure and record the electrical conductivity (EC) of the water using an EC meter as a rough check for consistency. If the EC changes seasonally, it may be advisable to order another water analysis so you know what's going on.
-
If your water already contains calcium and other minerals, then subtract these concentrations from your recipe's nutrient requirements.
5 helpful tips on mixing water soluble fertilizer
-
Use a sterile stirring stick of an inert material (e.g., an aluminum or plastic rod, but not wood) and a clean tank.
-
Thoroughly dissolve the fertilizer in a jug before adding it to your larger tank for easier mixing.
-
If you are mixing together more than one fertilizer, be careful about combining certain compounds that can precipitate in high concentrations. For example, one general rule is to keep stock solutions with calcium in a separate tank from phosphates and sulfates.
-
Measure and manage the pH after adding fertilizer.
-
Use an opaque container or store your fertilizer solution away from light to prevent algae from growing. If the fertilizer solution tank is not opaque, cover the outside with opaque plastic or foil.
Converting concentrations from mmol to ppm
Fertilizer recipes may call for concentrations in molarity units, such as millimoles per liter (mmol/L) or micromoles per liter (µmol/L) instead of ppm. Molar units are especially common in European countries and are also useful for understanding chemical reactions.
A mole is 6.023 × 10²³ units or particles of something (This is known as Avogadro's number.), so a mole of nitrogen represents 6.023 × 10²³ nitrogen atoms.
To convert molar units (number per volume) of an element or compound to ppm (weight per volume), you need to know the molar mass of the element or compound. Note that the molar mass of an element is the same as its atomic weight. The equation to convert mmol/L to ppm is:
ppm (mg/L) = molar concentration (mmol/L) × molar mass (g/mol)
How much calcium nitrate would you need to achieve a concentration of 5.4 mmol calcium per liter?
Since the molar mass of calcium is 40.078 g/mol, the required ppm of calcium is:
5.4 mmol/L × 40.078 g/mol = 216.4 mg/L
Next, if the calcium nitrate fertilizer contains 19% calcium by weight, you can calculate how much calcium nitrate to add:
216.4 mg/L calcium / 0.19 = 1139 mg/L calcium nitrate
Divide the answer by 1000 mg/g to get the final answer of 1.1 g/L of calcium nitrate.
Thus, to achieve a concentration of 216.4 ppm calcium, add 1.1 g/L of calcium nitrate. Try using the fertilizer ppm calculator to check the answer!
What if I want to use tablespoons to measure fertilizer?
If you're growing plants at home as a hobby, you might not have a kitchen scale or a desire for perfect accuracy. While estimating fertilizer volume is not adequate for commercial growing, it's possible to roughly estimate how much fertilizer to add in terms of tablespoons (1 tbsp = 15 mL) for the casual hobbyist (but still not highly recommended). This method only applies to "complete fertilizers", which contain a pre-made mix of macro- and micro-nutrients. Don't use volume estimation when creating a custom fertilizer solution from various compounds.
However, proceed with caution. It is crucial to recognize that volume estimation of fertilizer is usually inaccurate. Depending on how tightly packed the fertilizer is, the amount of fertilizer you add each time will change. To see this phenomenon, use a spoon to fill a small plastic cup about halfway with fertilizer. Mark the fill line with a marker. Then, gently drop the cup (vertically!) from about 1 inch off the table three times. How much did the fill-line change?
The key to estimating fertilizer volume is to recognize that every fertilizer has a different density. You will need to know the bulk density for your specific fertilizer, which you can find on the Safety Data Sheet (search for "SDS" or "MSDS") for your fertilizer. Every company that sells fertilizer should have published an SDS to go along with the product, but you might have to conduct a web search to find it. For example, a brief survey of water-soluble fertilizers had bulk densities ranging from 0.7 kg/L to 1.4 kg/L.
You'll still have to find the SDS sheet to find the bulk density of your fertilizer, then divide your required fertilizer weight by the bulk density in order to get volume:
fertilizer volume = fertilizer weight / bulk density
We do hope that after you've developed a green thumb, you'll eventually want to pick up a tabletop weighing scale for more consistent results!