Molarity Calculator
This molarity calculator is a tool for converting the mass concentration of any solution to molar concentration (or recalculating grams per ml to moles). You can also calculate the mass of a substance needed to achieve a desired molarity. This article will provide you with the molarity definition and the molarity formula.
To understand the topic as a whole, you will want to learn the mole definition, read a paragraph about the molarity units, as well as read a comparison of two misleading concepts: molarity formula vs molality formula. What is more, we prepared for you some interesting examples of molar solutions and a short step-by-step tutorial on how to calculate the molarity of a concentrated solution.
At the end, you can learn the titration definition and discover how to find the molar concentration using the titration process!
How to use the molarity calculator
The molarity calculator is straightforward and convenient, and you will find that out soon. But did you know how significant calculating molarity is? Keeping product quality and compliance with safety regulations requires determining the molarity of specific compounds or additives in the food and beverage industry.
-
If you know the concentration of the solution, enter it into the 4th variable of the calculator. One interesting fact is that the mass concentration is equal to its density for a pure substance. The default unit here is g/mL, grams per milliliter, but you can change it from the given options; make sure to change the unit before you enter the value.
-
Next, input the molecular weight (or molar mass) of the substance. The default unit is grams per mole, but you can change it first to other commonly used units.
-
Now, see the magic happen as the calculator instantly determines the molarity. The default unit here is molars (M). You can change it per your requirement from the given list of options.
-
But what do you do if you do not have the mass concentration of the substance? How to calculate the molarity now? Our tool has the answer for that as well. In this situation, leave the mass concentration empty.
-
Input the mass of the substance in grams or change the unit if needed.
-
Lastly, enter the volume of the solution.
-
Our tool is smart enough to determine the mass concentration and molarity based on the information you provided.
Let's consider an example. Your desired substance is sulphuric acid, and the molar mass is 98 g/mol with a mass concentration of 10 g/ml. Entering these values in the calculator will give you the molarity as 102.0408 M.
Now, imagine you did not know the mass concentration of the acid. Instead, you have the mass and volume of the solution as 970 g of H2SO4 in a 2.1 L solution. The molarity calculator will tell you that the molarity of your acid is 4.71331 M, with the mass concentration being 0.461905 g/ml.
Molar concentration – an introduction
When you look around, even if you're sitting at home, you will notice many different objects. The majority of these materials are not pure. They are, in fact, mixtures.
Mixtures consist of a collection of different compounds. Occasionally, the number of elements may be quite high, or sometimes quite low, but as long as there is more than one element in an object, it is a mixture. Orange juice in your glass, a cup of tea, detergents in the bathroom or milk – all these substances are mixtures.
Mixtures are not limited to just liquids though, solids and gases can both be mixtures; even biological organisms are very complex mixtures of molecules, gases, and ions dissolved in water.
In chemistry, there are two types of mixtures:
-
Homogeneous mixtures – Components are uniformly distributed throughout the mixture, and there is only one phase of matter observed. They are also known as solutions and may occur in the solid, liquid or gaseous state. It is not possible to simply separate the mixture components, but no chemical change has occurred to any of the components. Examples: sugar water, dishwashing detergent, steel, windshield washer fluid, air.
-
Heterogeneous mixtures – Components of the mixture are not uniformly distributed and may have regions with different properties. Different samples of the mixture are not identical. At least two phases are always present in the mixture, and it's usually possible to physically separate them. A few examples of such substances: blood, concrete, ice cubes in cola, pizza, the Pacific Ocean.
Concentration is one of the most well known and most important parameters for anybody who works with any chemical substances or reactions. It measures how much of a substance is dissolved in a given volume of solution.
Chemists use many different units for describing concentration. However, the term molarity, also known as molar concentration, is the most common way of expressing the concentration. When the reactants (compounds) are expressed in mole units, it allows them to be written with integers in chemical reactions. This helps to easily work with their amounts. First, let's take a closer look at what is the mole, so we can move on later to find what is molarity.
Mole definition
The mole is the SI unit of measurement for the amount of substance. The current definition was adopted in 1971 and is based on carbon-12. It says:
"The mole is the amount of substance of a system which contains as many elementary entities as there are atoms in 0.012 kilograms of carbon-12; its symbol is "mol". When the mole is used, the elementary entities must be specified and may be atoms, molecules, ions, electrons, other particles, or specified groups of such particles."
It follows that the molar mass of carbon-12 is exactly 12 grams per mole, M(¹²C) = 12 g/mol. The word "substance" in the definition should specify (be replaced with the name of) the substance concerned in a particular application, e.g., the amount of chloride (HCl) or the amount of carbon dioxide (CO₂). It is crucial to always give a precise specification of the entity involved (as noted in the second part of the mole definition). This should be done by providing the empirical chemical formula of the compound involved.
According to the newest conventions (effective as of the 20th May 2019), the mole definition is that a mole is the amount of a chemical substance that contains exactly 6.02214076 × 1023 particles, such as atoms, molecules, ions etc. That number is known as Avogadro's constant. Its symbol is NA or L. Using the Avogadro number provides a convenient way of considering the weight of substance and the theoretical yield of chemical reactions. Moles allow you to directly read weight from the periodic table (e.g., 1 mole of N₂ is 28 g or 1 mole of NaCl is 58.5 g).
We can link the number of entities X in a specified sample – N(X), to the moles of X in the same sample – n(X), with the relation: n(X) = N(X)/NA. N(X) is dimensionless, and n(X) has the SI unit mole.
What is molarity?
So you are not confused with similar chemical terms, keep in mind that molarity means exactly the same as molar concentration (M). Molarity expresses the concentration of a solution. It is defined as the number of moles of a substance or solute, dissolved per liter of solution (not per liter of solvent!).
concentration = number of moles / volume
Molarity formula
The following equation allows you to find the molarity of a solution:
molarity = concentration / molar mass
The concentration denotes the mass concentration of the solution, expressed in units of density (usually g/l or g/ml).
Molar mass is the mass of 1 mole of the solute. It is expressed in grams per mole. It is a constant property of each substance – for example, the molar mass of water is approximately equal to 18 g/mol.
Our calculator can also find the mass of substance you need to add to your solution to obtain a desired molar concentration, according to the formula:
mass / volume = concentration = molarity × molar mass
where mass is the mass of solute (substance) in grams, and volume is the total volume of solution in liters.
🔎 Molarity has many applications. One of them is calculating the solution dilution. Learn more in the solution dilution calculator.
Molarity units
The units of molar concentration are moles per cubic decimeter. They are noted as mol/dm³ as well as M (pronounced "molar"). The molar concentration of solute is sometimes abbreviated by putting square brackets around the chemical formula of the solute, e.g., the concentration of hydroxide anions can be written as [OH⁻]. In many older books or articles, you can find different units of molar solutions – moles per liter (mol/l). Remember that one cubic decimeter equals to one liter, so these two notations express the same numeric values.
Formerly, chemists used to give concentrations as the weight of solute/volume. Nowadays, since mole has become the most common way of quoting the quantity of a chemical substance, molarity is commonly used instead.
Note that molarity might be quite often confused with the term molality. Molality is usually written with lower case m, while molarity (what was mentioned above) with an uppercase M. We explain the difference between these two in a paragraph below.
How to calculate molarity
- Choose your substance. Let's assume that it is the hydrochloric acid (HCl).
- Find the molar mass of your substance. For the hydrochloric acid, it is equal to 36.46 g/mol.
- Decide on the mass concentration of your substance. Let's assume you have 5 g of HCl in a 1.2 liter solution.
- Convert the expressions above to obtain a molarity formula. As
mass/volume = molarity × molar mass, thenmass / (volume × molar mass) = molarity. - Substitute the known values to calculate the molarity:
molarity = 5 / (1.2 × 36.46) = 0.114 mol/l = 0.114 M. - You can also use this molarity calculator to find the mass concentration or molar mass. Simply type in the remaining values and watch it do all the work for you.
Molarity vs. molality
Let's consider the differences between these two similarly named chemical concepts: molarity and molality. We hope that after reading this paragraph, you will have no doubts regarding this topic.
Both terms are used to express the concentration of a solution, but there is a significant difference between them. While molarity describes the amount of substance per unit volume of solution, molality defines the concentration as the amount of substance per unit mass of the solvent. In other words, molality is the number of moles of solute (dissolved material) per kilogram of solvent (where the solute is dissolved in).
It is possible to recalculate from molarity to molality and vice versa. To make this shift, use the formula below:
molarity = (molality × solution mass density) / (1 + (molality × solute molar mass))
In this molarity vs molality table, you can find all main differences between these two terms:
Molarity | Molality | |
|---|---|---|
Definition | Amount of substance (in moles) divided by the volume (in litres) of the solution. | Amount of substance (in moles) divided by the mass (in kg) of the solvent. |
Symbol | M | m or b |
Unit | mol/L | mol/kg |
Temperature and pressure | Dependent | Independent |
Usage | More popular, practical to use in the lab, faster and easier. | Accurate but rarely used. |
If you want to explore this subject further, feel free to refer to our article Molarity vs. Molality: Understanding the Key Differences.
Molar solution — life examples
As you already know, mixtures and solutions always surround us, and they are a permanent part of the environment. In the table below, you can find the list of orders of magnitude for molar concentration, with examples taken from the natural environment.
Molarity | SI prefix | Value | Item |
|---|---|---|---|
10⁻¹⁵ | fM | 2 fM | Bacteria in surface seawater (1×10⁹/L) |
10⁻¹⁴ | – | 50–100 fM | Gold in seawater |
10⁻¹² | pM | 7.51–9.80 pM | Normal range for erythrocytes in blood in an adult male |
10⁻⁷ | – | 101 nM | Hydronium and hydroxide ions in pure water at 25 °C |
10⁻⁴ | – | 180–480 µM | Normal range for uric acid in blood |
10⁻³ | mM | 7.8 mM | Upper bound for healthy blood glucose 2 hours after eating |
10⁻² | cM | 44.6 mM | Pure ideal gas at 0 °C and 101.325 kPa |
10⁻¹ | dM | 140 mM | Sodium ions in blood plasma |
10² | hM | 118.8 M | Pure osmium at 20 °C (22.587 g/cm³) |
10⁴ | myM | 24 kM | Helium in the solar core (150 g/cm³ × 65%) |
Determining the molar concentration by titration
Titration is a technique with which you can find the concentration of an unknown solution, based on its chemical reaction with a solution with a known concentration. This process is based on adding the titrant (with a known concentration & volume) to a known quantity of the unknown solution (the analyte) till the reaction is complete. You can then determine the concentration of the analyte by measuring the volume of titrant used.
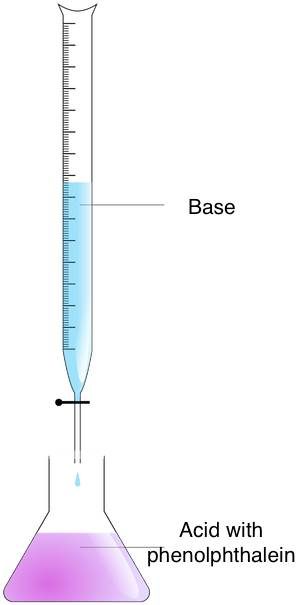
Follow these steps to find the molarity of an unknown solution with the titration method:
- Prepare the concentrations – Put the analyte in an Erlenmeyer flask and the titrant in a burette.
- Mix the concentrations – Add the titrant to the analyte until the endpoint is reached. You can find this moment by observing the color change. Use the acid-base indicator for this purpose. If you have used phenolphthalein, you will notice a color change from pink to colorless.
- Calculate the molarity – Use the titration formula. If the titrant to analyte ratio is 1:1, use the equation:
acid molarity × acid volume = base molarity × base volume.
For ratios other than 1:1, you need to modify the formula.
🙋 Learn how to calculate titrations in the titration calculator.
Example: 35 ml of 1.25 M HCl acid is needed to titrate a 25 ml solution of NaOH. In that case, you can use the 1:1 formula because one mole of HCl reacts with one mole of NaOH. Then, multiply the molarity of the acid by the volume of the acid – 1.25 × 35 = 43.75 and the result, by the volume of the base. The molarity of the base equals 43.75 / 25 = 1.75 M.
🔎 Make sure you check out our alligation calculator if you are interested in determining how to obtain different concentrations of a solution.
Invention of the molarity calculator
You can understand the significance of the molarity calculator because two of our top brains collaborated to bring this convenient tool into existence.
Bogna Szyk's professional and personal journey is a testament to her multifaceted talents and dedication. Her love for developing practical tools, from calculators to Notion databases, showcases her technical proficiency and desire to share knowledge and tools for the betterment of others.
Filip Derma is a distinct and credible figure in biomedical and electrical engineering. His willingness to embrace various subjects further underscores his versatility and eagerness to engage with diverse challenges, showcasing a commendable intellectual curiosity and adaptability.
Filip and Bogna collaborated to create this tool, realizing that molarity calculations could be tedious and that having a molarity calculator would be a handy teaching aid, helping students grasp concepts faster and apply them in practical scenarios without getting confused by the arithmetic.
We ensure that our tools provide the correct answer and invest a significant amount of research and effort in ensuring that authentic information is provided to our users, which they can apply in both educational and practical scenarios. To learn more about our commitment to quality, please refer to our Editorial Policies page.
FAQs
How do I calculate pH from molarity?
- Calculate the concentration of the acid/alkaline component of your solution.
- Calculate the concentration of H+ or OH- in your solution if your solution is acidic or alkaline, respectively.
- Work out -log[H+] for acidic solutions. The result is pH.
- For alkaline solutions, find -log[OH-] and subtract it from 14.
How do you make a molar solution?
- Find the molecular weight of the substance you’d like to make a molar solution of in g/mol.
- Multiply the molecular weight of the substance by the number of moles you wish to have, which in this case is 1.
- Weigh out the number of grams you calculated in step 2 of your substance and place it in a container.
- Measure out 1 liter of your chosen solvent and add it to the same container. You now have a molar solution.
What is molar volume?
Molar volume is the volume that one mole of a substance takes up at a particular temperature and pressure. It is found by dividing the molar mass by the substance’s density at that temperature and pressure.
How do I find moles from molarity?
- Find the molarity and volume of your solution.
- Make sure that the units for the volume are the same as for the volume part of the molarity (e.g., mL and mol/mL).
- Multiply the volume by the molarity. This is the number of moles present.
Is molarity the same as concentration?
Molarity is not the same as concentration, although they are very similar. Concentration is a measure of how many moles of a substance are dissolved in an amount of liquid, and can have any volume units. Molarity is a type of concentration, specifically moles per liter of solution.
What is the molarity of water?
Water has a molarity of 55.5 M. 1 liter of water weighs 1000 g, and, as molarity is the number of moles per liter; finding the molarity of water is the same as finding the number of moles of water in 1000 g. We therefore divide the weight by the molar mass to get moles, 1000 / 18.02 = 55.5 M.
Why do we use molarity?
Molarity is a helpful measure to use when discussing concentration. As concentration has a large range of sizes of units, from nanogram per milliliter to ton per gallon, it is easier to have a known metric for quick comparison of concentrations without having to deal with conversions. This is molarity (M), which is moles per liter.