Beer-Lambert Law Calculator
Omni's Beer-Lambert law calculator allows you to calculate the absorbance (or attenuation) of light as it passes through any material. You can also use this calculator to determine the molar concentration of solutions. Read on to know what Beer's law is and the formula for Beer's law calculations. You will also find how to calculate concentration from absorbance in Beer's law.
What is Beer's law (Beer-Lambert law)?
is also known as Beer's law or Beer-Lambert–Bouguer law. It gives a relationship between the concentration of a solution and the attenuation of light as it passes through the solution.
Beer's law states that when a beam of electromagnetic radiation passes through a sample (usually a solution), its absorbance depends on the concentration of the sample and the path length of the beam in the sample.
If you want, you may consult our concentration calculator for converting the molarity of a substance into percentage concentration.
Beer's law equation
To understand absorbance, let us consider figure 1. A light beam of intensity passes through a solution placed in a container of diameter .
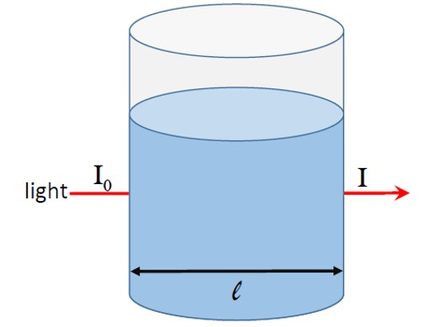
If the solution absorbs light, the intensity of the light emerging from the container will be less than . If the intensity of the transmitted light is , we can define the absorbance as:
We can express Beer's law equation as:
where:
- -Molar absorption coefficient or the molar absorptivity;
- -Path length of the beam in the sample; and
- -Concentration of the solution.
In spectroscopy, the path length is usually expressed in cm, and absorbance is unitless (a dimensionless quantity). The unit for expressing the concentration of sample solution is mol/L, and hence the units of molar absorptivity are L/mol·cm.
Might we recommend trying out our molarity calculator? It helps convert the mass concentration of any solution to a molar concentration.
How to use the Beer-Lambert law calculator
Let us see how to use Beer's law calculator to calculate the absorbance of light by a solution of molar concentration . Let the path length be , and the molar absorptivity is .
- Enter the value of the molar absorption coefficient, i.e., .
- Type the concentration of the solution, i.e., .
- Input the path length, i.e., .
- The calculator will display the absorbance: .
- You can also use this Beer-Lambert law calculator as a transmittance to absorbance calculator. Just enter the transmittance values in percentage, and you will get the absorbance.
Absorbance to transmittance conversion
In figure 1, the fraction of light that passes through the sample gives the transmittance T, i.e.:
…and we can express the relation between transmittance and absorbance as:
As we note, the relation between absorbance and transmittance is logarithmic. An absorbance of 0 implies a transmittance of 100%.
In spectrophotometry techniques, we measure the intensity of the radiation entering the sample solution and the intensity of radiation coming out of it. We then use the two intensities to calculate the transmittance or absorbance values.
Applications of the Beer-Lambert law
Most of the spectroscopic analysis techniques in chemistry are based on the Beer-Lambert law. Some common applications of Beer's law in analytical chemistry are:
- To determine the concentration of samples by measuring the absorbance.
- To determine the identity of an unknown substance by determining its molar absorptivity.
We have a tool that helps you calculate the proportions of two solutions to be mixed in order to produce a required solution with a specific concentration, the alligation calculator.
FAQs
What is the unit of absorbance in Beer's law?
The absorbance is a unitless quantity. It is the ratio of the intensity of the incident light and the transmitted light; hence, it is dimensionless and has no units. However, sometimes absorbance is reported in absorbance units (AU).
How do I calculate molar absorptivity from Beer's law?
To calculate molar absorptivity from Beer's law, proceed as follows:
- Multiply the path length with the molar concentration of the solution.
- Divide the absorbance by the value obtained in step 1.
- Congrats! You have successfully calculated the molar absorptivity from Beer's law.
How do I calculate concentration from absorbance in Beer's law?
Beer-Lambert law is often used in determining the concentration of solutions. To calculate the concentration of a solution from Beer's law, follow the given instructions:
- Determine the absorbance as the light of a given wavelength passes through the solution.
- Find out the path length the light has to travel.
- Multiply the molar absorption coefficient with the path length.
- Divide the absorbance by the value obtained in step 3, and you will get the concentration of the solution.
How do I calculate transmittance from absorbance?
To calculate transmittance from absorbance, we need to follow the given steps:
- Subtract the absorbance value from the number
2. - Take the antilog of the value obtained in step 1, and you will get the transmittance percentage.
- You can also use our Beer-Lambert law calculator to calculate transmittance.