Freezing Point Depression Calculator
Using Omni's freezing point depression calculator, you can estimate the change in the freezing point temperature of a solution compared to the pure solvent.
Ever wondered why we use common salt to de-ice roadways and sidewalks during winter? How does adding a solute affect the freezing point of a solution (or a solvent)? If you have - continue reading to understand what is freezing point depression and how to calculate it using our freezing point depression calculator. You will also find some applications of the phenomenon in everyday life.
What is freezing point depression?
When we add a nonvolatile solute to a volatile solvent, the solution's freezing point always becomes lower than that of the pure solvent. This decrease in the freezing point of the solution compared to that of the pure solvent is called the freezing point depression.
The extent to which the freezing point decreases depends on the concentration of the solution.
Freezing point depression formula
According to Raoult's Law, the vapor pressure of a solution is always lower than that of the pure solvent.
In the freezing point depression graph shown in Figure 1:
- Curve BC represents the vapor pressure of the pure solvent;
- Curve DE represents the vapor pressure of the solution; and
- Curve AB represents the vapor pressure of the solid (frozen state).
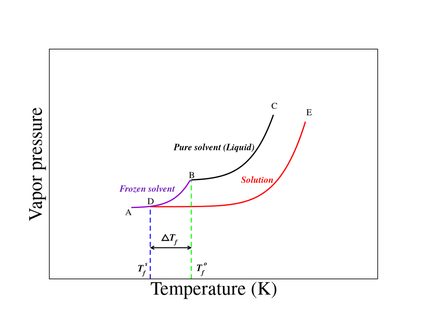
The freezing point is the temperature at which the solid and liquid states of a substance have the same vapor pressure.
🔎 Learn more in the vapor pressure calculator.
From the graph, we note that:
- (temperature corresponding to point B) represents the freezing point of the pure solvent; and
- (temperature corresponding to point D) represents the freezing point of the solution.
Due to the lowering of vapor pressure, the freezing point of solutions decreases with the addition of nonvolatile solute.
We can express the change or depression in freezing point as:
The depression in freezing point is directly proportional to the molal concentration of the solution :
, where is the molal freezing point depression constant or cryoscopic constant.
We call the above equation the freezing point depression formula.
How to calculate freezing point depression?
Let us calculate the freezing point of a solution of ethylene glycol in water using our freezing point depression calculator. A solution is a solution that contains 1 gram mole of solute per 1000 g of solvent.
-
Enter the molality of the solution, i.e., . You can use the molality formula to find the molality of the solution.
-
Choose the solvent from the drop-down menu. The calculator will autofill the freezing point depression constant and the freezing point for the solvent of your choice. If you already know these values, you can proceed to the next step.
-
Input the value of molal freezing point depression constant for water, i.e., .
-
Type the freezing point of pure water, i.e., .
-
The calculator will display the freezing point of the solution, i.e., .
-
You can also open the last section of this calculator to input the van't Hoff factor for the solute.
We also recommend checking out our boiling point at altitude calculator to explore the effect of altitude on boiling point.
The van't Hoff factor
The freezing point depression formula discussed so far, is valid only for nonelectrolyte solutes, i.e., solutes that dissolve without any dissociation. However, when we dissolve electrolytes like common salt (sodium chloride, NaCl) they undergo dissociation in the solution according to the reaction:
If we dissolve of NaCl in of water, we will get of Na+ and of Cl- ions i.e., of ions. This of course is true only when it undergoes complete dissociation, however, experimental observations have revealed that the ions of sodium chloride do not undergo complete dissociation in solution.
To account for this, an additional experimentally measured parameter is used in the formula for freezing point depression:
This additional term called the van't Hoff factor (i), is defined as:
The measured value of van't Hoff factor for NaCl is .
Molal freezing point depression constant for some solvents
According to the formula for freezing point depression, for , . Thus, the molal freezing point depression constant is the depression in the freezing point for a solution. The units of are and .
Take a look at the table below to see the molal freezing point depression constant for water and some common solvents.
Solvent | Freezing point (°C) | Kf (°C·kg/mol) |
|---|---|---|
Water | 0.0 | 1.86 |
Benzene | 5.5 | 5.12 |
Ethanol | -114.6 | 1.99 |
Chloroform | -63.5 | 4.68 |
Ether | -116.2 | 1.79 |
Examples of freezing point depression
Some common examples of the freezing point depression phenomenon and its application in everyday life are:
-
Salting of roads in winter: In winter, we use common salt (NaCl) or calcium chloride (CaCl2) to clear the ice on the roads. This is because mixing salt with water lowers the freezing point of water below 0 °C. As a result, water would not freeze at low temperatures, and the roads will be free from ice.
-
Anti-freeze solutions: Automobile radiators use a mixture of ethylene glycol in water to lower the freezing point of water. This avoids the freezing of water in the radiators at places where the temperature is less than zero centigrade.
-
Ice cream: The sugar in ice cream mix lowers the freezing point of water. As a result, ice cream is not completely frozen like a block of ice at serving temperatures. This allows you to scoop and eat it.
FAQs
What is the freezing point?
The freezing point is the temperature at which a substance changes its physical state from liquid to solid. At the freezing point, the substance's vapor pressure in its liquid phase is equal to the vapor pressure in its solid phase.
Is freezing point a chemical property?
No. The freezing point of a substance is a physical property. The physical state of water changes from liquid (water) to solid (ice) during freezing. The chemical properties of water (H2O) don't change.
What is the freezing point of water in Celsius?
The freezing point of water in Celsius is 0 °C. If we add a nonvolatile solute like common salt to the water, the solution's (salt + water) freezing point becomes lower than 0 °C.
What is the freezing point of water in Kelvin?
The freezing point of water in kelvin is 273.15 K. The relation between Celsius and Kelvin scales of temperature is TKelvin = TCelsius + 273.15. The freezing point of water in Celsius is 0 °C; hence, the freezing point is 273.15 K in the Kelvin scale.
How to determine molar mass from freezing point depression?
To determine molar mass from freezing point depression, we will follow the given steps:
-
Dissolve a known mass (wb grams) of the solute in a known mass (wa grams) of a solvent.
-
The molality m of this solution is:
m = (wb * 1000) / (Mb * wa);
where Mb is the unknown molar mass of the solute. -
Substitute the value of m into the freezing point depression equation, and rearrange to get: Mb = (Kf * wb * 1000) / (ΔTf * wa).
-
Now, all you have to do is to measure the freezing point depression ΔTf, and you can determine the molar mass of any solute using the above equation.